Hucord’s Technology
Since establishing its cord blood bank in 2000, Hucord has played a leading role in the field of cord blood stem cells.
Hucord has proven its technology and reliability by being the first in the world to discover cord blood mesenchymal stem cells,
perform stem cell procedures on humans, hold key patents, and accumulate over 2,000 clinical cases.
World-First Achievements by Hucord
-
Proof of the Existence of Mesenchymal Stem Cells in Cord Blood
-
Disclosure of Cord Blood Hematopoietic Stem Cell Information
-
Various Differentiation Technologies Using Cord Blood Mesenchymal Stem Cells
-
Isolation and Culturing of Mesenchymal Stem Cells from Frozen Cord Blood
-
Allogeneic Cord Blood Mesenchymal Stem Cell Therapy
-
Successful Results Announced in the Treatment of Various Intractable Diseases, Including Spinal Cord Injury, Stroke, and Buerger’s Disease
Clinical Trials
Buerger’s Disease
-

Before
-

30 Days Post-Treatment
-
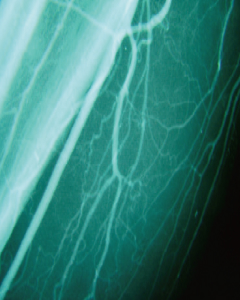
120 Days Post-Treatment
- Improvement of Lower Limb Blood Flow
- After treatment, new blood vessels formed in the calf muscle area, and the blood flow improved.
-
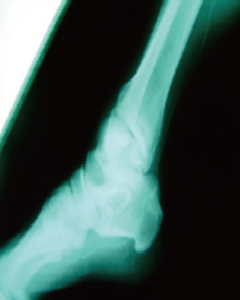
Before
-
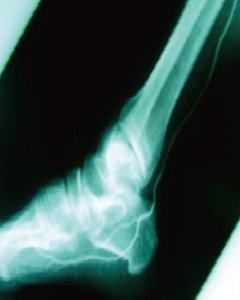
30 Days Post-Treatment
-
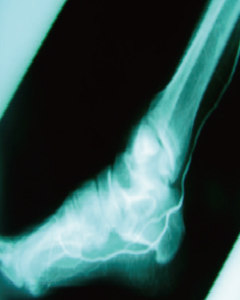
120 Days Post-Treatment
- Improvement of Foot Blood Flow
- After treatment, new blood vessels formed in the foot and calf areas, leading to improved blood flow.
Liver Cirrhosis
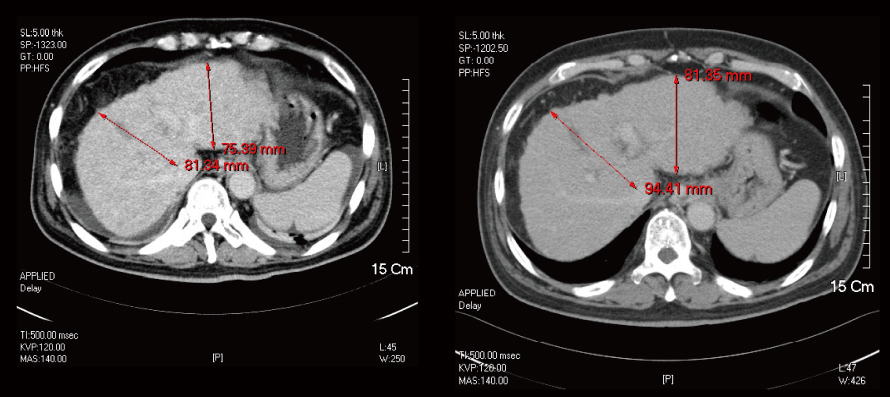
The liver’s size recovered from 598.98 ml to 915.36 ml.
Stroke
-
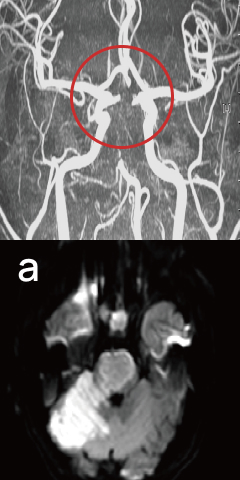
At Onset
-
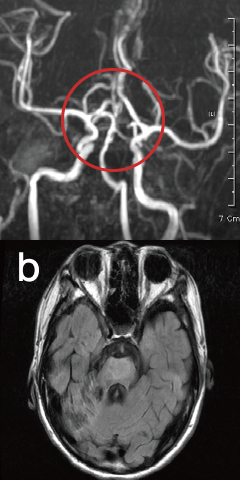
15 Days Post-Treatment
-
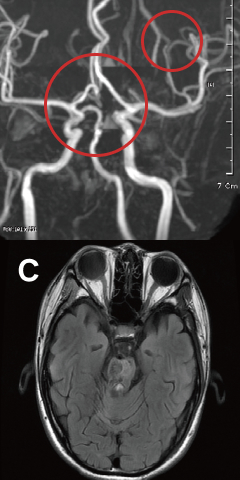
41 Days Post-Treatment
New blood vessels have formed around the ruptured brain vessels.
Paraplegia
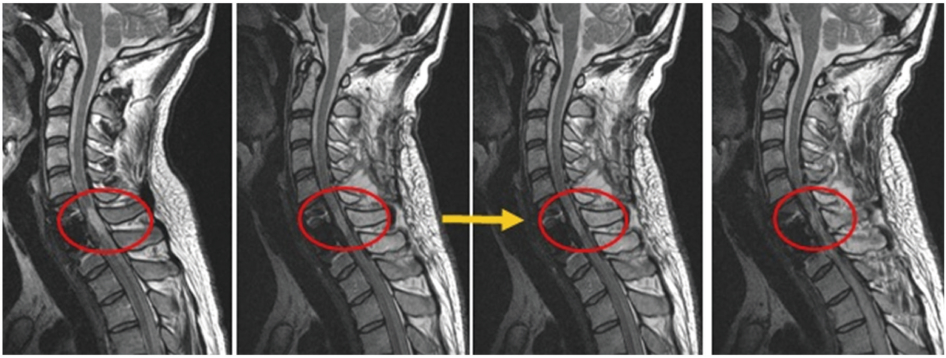
The MRI shows that the lesion area is gradually becoming darker, indicating improvement in the affected region.
Osteoporosis
-
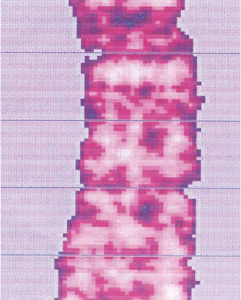
Before
-
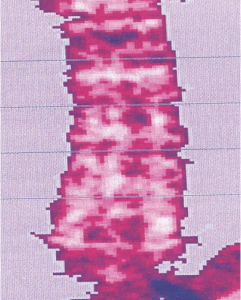 BMD 0.5601g/cm² BMD 0.5918g/cm²
BMD 0.5601g/cm² BMD 0.5918g/cm²After
-
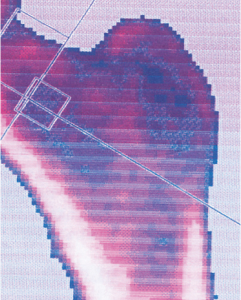
Before
-
 BMD 0.5217g/cm² BMD 0.5309g/cm²
BMD 0.5217g/cm² BMD 0.5309g/cm²After
In cases of osteoporosis, stem cell therapy has shown a significant increase in bone density.
